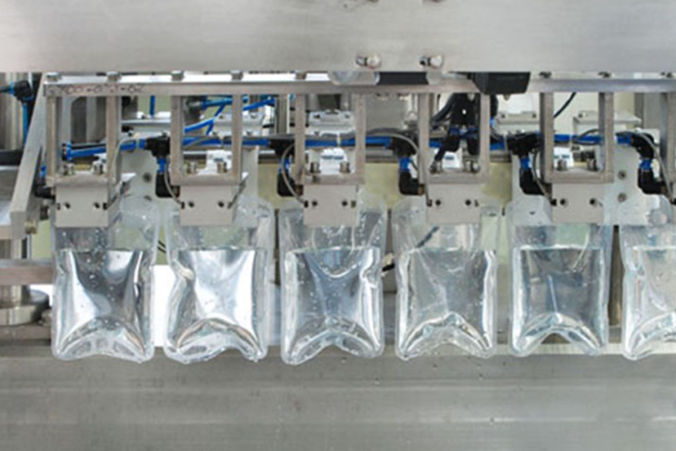
Machine

Infusion Bag

We are establishing cGMP-compliant formulation manufacturing facility, with a well-structured Quality Control lab to ensure high product standards. This facility will manufacture a wide range of injectables using aseptic filling / terminal sterilization, as applicable.
Lillington, NC
55,000-sft flagship facility with world-class infrastructure to make a wide variety of liquid injectables.
Bag Filling line (50ml to 1000ml): 15 million units / year


